Annual consortium meeting,Hawassa
Hawassa, Ethiopia – August 6, 2024 – The Annual PREGART Project Consortium meeting took place from August 5-6, 2024, at Haile Resort in Hawassa City, Ethiopia.
The event brought together over 60 participants, including consortium members, government stakeholders, and representatives from the Ministry of Health, the Ethiopian Food and Drug Authority, and partner organizations. Attendees gathered to review the project’s progress, share insights, and outline plans for the year ahead.
The meeting began with a welcoming address by Dr. Ayano Beraso, President of Hawassa University, followed by keynote speeches from representatives of the European & Developing Countries Clinical Trials Partnership (EDCTP) and the Ministry of Health of Ethiopia. The speakers highlighted the crucial role of collaboration, innovation, and effective communication in achieving the objectives of the PREGART Project. The opening session of the PREGART Project meeting provided a thorough overview, covering project progress, challenges encountered during the COVID-19 pandemic, and strategies adopted to overcoming them. The workshop served as a platform for participants to reflect on the accomplishments of the past year, exchange
best practices, and identify areas for further improvement. Subsequent closed sessions for consortium members from Ethiopia, Uganda, and Sweden focused on status of study participants recruitment and follow-up, data management and analysis methods, and preliminary findings. Four PhD students presented updates on their ongoing research followed by interactive discussions and
guidance to sustain their momentum in their respective studies.
The workshop celebrated achievements and tackled project challenges, concluding with a renewed commitment to the project’s goals and a focus on continuous improvement and collaborative success.
The PREGART Project is dedicated to enhancing antiretroviral treatment outcomes for pregnant women and improving prevention of mother-to-child transmission (PMTCT) across Africa. The PREGART Project remains dedicated to fostering collaboration, driving
innovation, and advancing research to improve antiretroviral treatment outcomes for pregnant women and enhance prevention of mother-to-child transmission (PMTCT) outcomes across Africa.
The PREGART project is part of the EDCTP2 Programme supported by the European Union.

Trial Site Technical Support
The PREGART project principal investigators (PIs) and PhD students’ supervisors, Professor Eleni Aklilu and Professor Eyasu Makonnen, paid a visit to the Ethiopian PEGART Clinical Trial Site in February 2024. The purpose of the visit was to monitor the trial activities and progress of the PhD students. During the events, PhD students presented the status of their dissertation work and discussed. The overall progress of the PREGART clinical trial was discussed with Hawassa University’s president. Directions were given to solve the identified challenges of the project.


Amended Protocol Popularization Workshop
The PREGART clinical trial project conducted an amended protocol popularization workshop from March 15–16, 2024. The PREGART Project Office in Ethiopia organized the workshop to clarify some amendments approved by the regulatory bodies for implementation. Twenty-six participants (health professionals participating in the trial at the study hospitals and focal persons from regional health bureaus) participated in the workshop. The main issues discussed included the dropping of the EFV 600 arm, the no-cost extension of the trial for an additional 18 months, inclusion and exclusion criteria, informed consent, and clinical and laboratory schedules. Moreover, CRF completion and data quality management, including problems identified during monitoring of the trial, were discussed.


Clinical Trial Methodology and Interpretation - Ethiopia
The Pregart clinical trial project conducted training on Clinical Trial Methodologies for trial staff working at study hospitals from March 20-22, 2023. The Pregart project office in Ethiopia organized the training as part of capacity building for stakeholder collaborating with the implementation of the trial. Over 20 physicians, antiretroviral therapy (ART) focal persons from Ministry of Health and 4 regions, participated in the three days training.



Capacity building: PhD and postdoctoral careers in the Pregart clinical trial
Hawassa University PREGART clinical trial project PhD candidates presented their individual study plan (ISP) to the postgraduate admission committee at KI, Sweden
One of the PREGART clinical trial project deliverable is deliverables is capacity building in Africa. Accordingly, one of the project consortium institution, Karolinska Institute (KI) in Sweden has been enrolling 4 PhD and 2 -Post doc students from Hawassa University and Makerere University with a primary advisor of Professor Eleni Aklilu. Two of the PhD candidates from Hawassa university; Mr. Siraj Hussein with the title “ Safety, Pharmacokinetics, and Pharmacogenetics of ART in pregnant and breastfeeding women in Ethiopia” from the Department of Medical Laboratory Science and Dr. Abel Gedefaw with the title “Optimization of Antiretroviral Treatment regimens for HIV-infected pregnant and breast-feeding women in Ethiopia” from the School of Medicine presented their individual study plan for the admission committee at KI on February 16 /2023. Two of the PhD students from Makerere University are going to present their study plan before the end of March 2023. The ISP is a governing document for the entire education and should ensure that the education is performed, and followed-up, in an efficient way. All the PhD students are expected to complete their project before spring 2026.


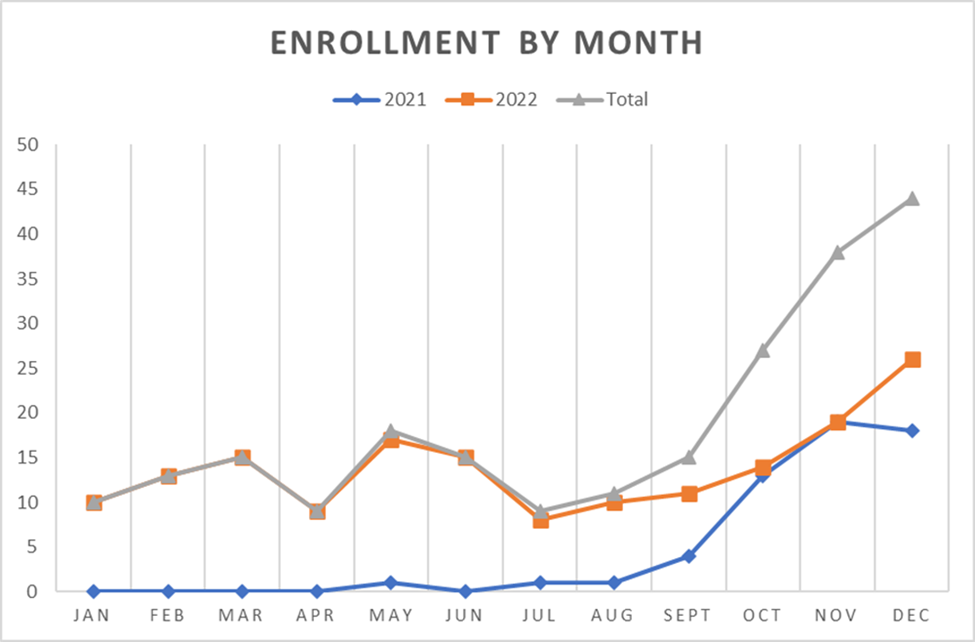
PREGART clinical trial in Ethiopia achieved 25% of the participant’s recruitment
The PREGART Clinical trial in Ethiopia has been enrolling participants since May 2021. The participant recruitment was initiated after 18 months of delay from the initially planned date due to the Covid-19 pandemic’s impact on the regulatory approval and investigational drug procurement process. Moreover, the recruitment rate was further affected by the Northern Ethiopia war. Three well-prepared trial hospitals for recruitment were canceled due to the ongoing war in the districts where the hospitals were located. However, amendment approval was secured on September 2022 to increase the trial hospital numbers and the replacement of the northern hospitals with the central and southern hospitals to fasten the recruitment rate. Before the end of 2022, 229 (25%) participants were recruited. The implementation team designed different strategies like primary health care facility-trial hospital linkage to achieve 75% recruitment in 2023.
Ethiopia Food and Regulatory Authority (EFDA) conducted a GCP inspection on the PREGART clinical trial implementation
The Ethiopia Food and Regulatory Authority (EFDA) pharmacovigilance and clinical trial authorization unit conducted an inspection of the PREGART clinical trial implementation (GCP inspection) from December 26-28 /2022. The inspection objective was to ensure the trial implementation compliance with the regulatory authority guidelines for good clinical practice (GCP). The inspection was conducted after a week of the notification by two inspectors. The inspection started with an opening meeting whereby the inspectors described the purpose, methods, and procedures for the inspection. During the inspection process, the inspectors verified the trial is conducted in compliance with the granted authorization, the approved trial protocol, and good clinical practice (GCP) by carrying out observation of the trial and participants’ documents, observation of trial facilities, and interviewing study staff and investigators. After the end of the inspection, an exit meeting was conducted in the presence of investigators, study clinicians, and Hawassa University research directorate representatives. The inspectors explained their comments and feedback on their observations pending the formal inspection report after two weeks.


Good Clinical Practice (GCP), Good Clinical laboratory practice (GCLP) and Protocol familiarization training - Ethiopia
The PREGART Clinical trial project conducted its kick-off meeting in September 2019. However, the trial participant recruitment initiated after two years of delay due to the impact of Covid-19 and lack of clinical trial insurance provider for timely ethical approval. As a catch-up plan, the project office in Ethiopia has identified additional 9 study hospitals that fulfilled the eligibility criteria.
A two days training on protocol popularization, Good Clinical Practice (GCP) and Good Laboratory Practice (GLP) was given from September 3-4, 2022 for the newly added trial site staffs to equip them with the necessary knowledge and skills of clinical trial implementation according to the international standards.
Forty-five trial site staffs, with different professional categories (Obstetrician/ gynaecologist, PMTCT nurses, Laboratory technologist, Pharmacist) and who will be responsible for the site trial implementation participated in the two days training.



Stakeholders engagement meeting at Adama, Ethiopia
The Pregart Clinical trial in Ethiopia has been enrolling participants since May 2021. The project has enrolled 16% of its target as of June 2022. Recruitment of participants was supposed to start January 2020, but it was delayed due to Covid-19 pandemic and ethical approval process. As part of the mitigation plan to enhance the recruitment process, Hawassa University organized a one-day stakeholders engagement meeting on July 30, 2022 at Adama City, Hillside Hotel in Oromia region.
The meeting aimed at updating the stakeholders the progress of Pregart clinical trial progress (performance in terms of timeline and challenges).The meeting was held to enhance support and engagement of the stakeholders through discussions on the pertinent issues and challenges faced in the course of implementation of the trial that will facilitates the recruitment process to achieve the project objectives within the remaining timeline. The participants were identified based on their direct or indirect roles in creating awareness facilitating communications about the project across the existing structures of health delivery system. These include participants from the Ministry of Health, Regional Health Bureau of Oromia; Southern Nations, Nationalities and Peoples Region; Sidama Region; and Addis Ababa City Council.
Moreover, representatives from the National Ethics Committee, Ethiopian Public Health Institute, Addis Ababa Regional Laboratory, and prevention of mother to child transmission (PMTCT) coordinators took part in the meeting. Thirty-five participants attended the meeting. The trial investigators presented the progress of the trial, success and major challenges and finally all the participants discussed the identified challenges and recommended different interventions to be taken at the different level of healthcare system.



Pregart clinical trial situation in Uganda
The recruitment of trial participants on the Ugandan arm of the Pregart study kicked off on the 28th of September 2021 and it has been running to-date for approximately 17 weeks.
So far, the team has managed to enroll about 40 participants, of these, 3 have delivered, and all mothers and babies are faring well.
An unfortunate encumbrance in picking up recruitment numbers occurred mostly from the current global Covid-19 pandemic and the related setbacks. Solutions to mitigate this situation have been put in place for a hopefully better outcome. Aside from these overt challenges, the trial thus far is progressing steadily with a hope that it shall pick up to enable the team meet project’s end goals.